Using various surface sensitive techniques under in situ conditions where the chemical reactions are taking place, we aim to reveal the fundamental principles underlying the formation of nanostructures, and to build on this foundation to synthesize highly-efficient nanocatalysts with desired structure and properties. I shall use various model chemical systems including single crystals, oxide–metal interfaces, solid–liquid interfaces, and nanoparticles, as well as these research efforts to contribute to achieving the goal of the “Center for Nanomaterials and Chemical Reactions” (i.e., studying nanoscience and chemical reactions using basic science to uncover the atomic and molecular processes taking place on the surface of nanocatalysts and find comprehensive solutions for dealing with the environmental and energy-related problems that future generations face).
We carried out in situ measurements of the oxidation state of the nanoparticles with ambient-pressure XPS to elucidate a possible mechanism for changes in activity as the size and composition of the nanoparticles change. [Kamran Qadir, Sang Hoon Joo, Bongjin S. Mun, Derek R. Butcher, J. Russell Renzas, Funda Aksoy, Zhi Liu, Gabor A. Somorjai*, and Jeong Young Park*. Intrinsic Relation between Catalytic Activity of Oxidation on Ru Nanoparticles and Ru Oxides Uncovered with Ambient Pressure XPS. Nano Letters. 12, 5761–5768 (2012).]
We combined these experimental results with measurement of electrochemical activity for the smart design of catalytic materials. [Jae Yeong Cheon, Jong Hun Kim, Jae Hyung Kim, G. Kalyan Chakravarthy, Jeong Young Park* and Sang Hoon Joo*. Intrinsic Relationship between Enhanced Oxygen Reduction Reaction Activity and Nanoscale Work Function of Doped Carbons. Journal of the American Chemical Society. 136, 8875 (2014).]
Ambient-pressure STM and electrochemical STM have been installed in the “Surface Science under Reaction Conditions” Group, "Center for Nanomaterials and Chemical Reactions", IBS. Using ambient-pressure STM, thermal evolution of CO-induced surface restructuring has been successfully observed [J. Kim, M. C. Noh, W. H. Doh, and J. Y. Park* Journal of the American Chemical Society 138(4), 1110-1113 (2016).].
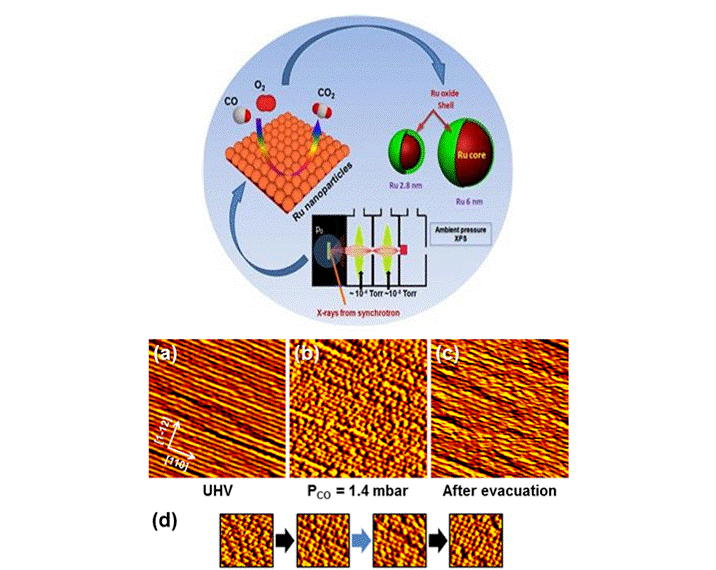
Figure.
In situ surface characterization of colloid nanoparticles (top) and AP-STM images showing the thermal evolution of CO-induced restructuring on Pt(557) (bottom).