Research Themes in Surface Science and Catalysis
As chemical energy conversion becomes the central theme of surface science research, our fundamental surface studies are moving to enable the evolution of new energy conversion technologies. For the past several decades, most surface science studies were carried out on single crystal surfaces under ultra-high vacuum conditions, while industrial catalysts are used at high pressures (including liquid phase) and with more complicated systems that consist of highly dispersed nanoparticles and oxide supports. In heterogeneous catalysis, the materials gap refers to the discontinuity between the well-characterized model systems and industrially relevant catalysts (Fig.1). In order to bridge the materials gap, it is crucial to develop new model catalysts, as described below.
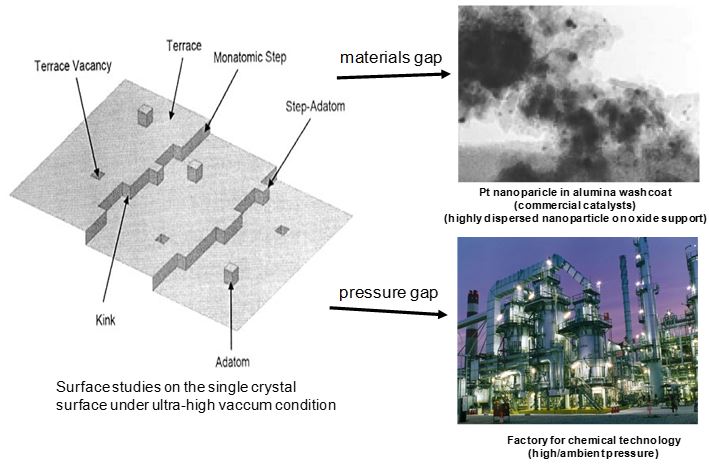
Figure 1 Schematic showing the pressure and materials gaps that have been central issues in surface science and heterogeneous catalysis.
In our group, we will focus on the fundamental atomic and molecular aspects of chemical reactions in complicated and realistic systems, which is a drastic transformation from the idealized model systems used in the past. We will use metal single crystals, oxide–metal interfaces, solid–liquid interfaces, and synthesize and fabricate metal nanoparticles. In situ experimental techniques capable of accessing different pressure regimes, from ultra-high vacuum to ambient pressure and solid–liquid interfaces, will also be employed. The new surface instruments will be used for atomic-level characterization of surfaces, including sum frequency generation (SFG) surface vibrational spectroscopy, ambient-pressure scanning tunneling microscopy (AP-STM), ambient-pressure atomic force microscopy (AP-AFM), and high-pressure X-ray photoelectron spectroscopy. As the frontiers of molecular surface science move from ultra-high vacuum surface studies of single crystals to high-pressure gas and solid–liquid interfaces to nanocrystals and nanocomposites, the new group will take the lead by developing prototype instruments for molecular surface studies of chemical structure, bonding, and reactivity. We have learned how to synthesize metal and nanocomposite nanoparticles, to characterize them, and to study their reactivities.
Bridging the Materials Gap - Development of Model Catalyst Systems
In the surface science community, new model catalysts have been developed over the past 10 years from studies of single crystal surfaces to nanoparticles fabricated by lithographic techniques and colloid synthesis. Single crystal surfaces served well as model catalytic systems that shed light on many surface phenomena. To consider factors including metal–support interactions and the importance of metallic cluster size, new catalytic model systems are now suggested. The phenomena associated with reduced dimensions, such as chemical activity variation due to metal nanoparticle size, shape, and composition, as well as the role of oxide–metal interfaces, are fundamental questions in surface chemistry and have been intensively studied. Colloid nanoparticles with stabilizing agents provide new opportunities to control the size and shape that are required to precisely quantify chemical influences. To fabricate two-dimensional (2D) or three-dimensional (3D) nanoparticle arrays, we deposit nanoparticle arrays on 2D supports or encapsulate them in 3D mesoporous supports, and carry out characterization and catalytic studies in these modes (Figure 2).
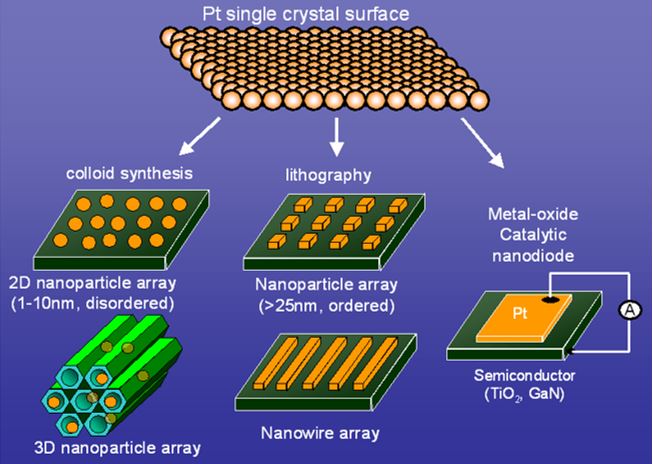
Figure 2 Schematic showing the evolution of the catalyst model system from a single crystal metal surface to 2D and 3D nanoparticle arrays
that are colloid synthesized to nanowire arrays and nanodiodes that are fabricated using lithography.
Bridging Pressure Gaps - Development of Novel in situ Surface Techniques
The most catalytically active systems are employed at high pressure or at solid–liquid interfaces. In order to study high pressure and liquid interfaces on the molecular level, experimental techniques bridging the catalysis pressure gap have been developed. These techniques include an ultra-high vacuum system equipped with a high-pressure reaction cell, high-pressure SFG vibration spectroscopy, AP-STM, AP-XPS, and atomic force microscopy (AFM), as shown in Figure 3.
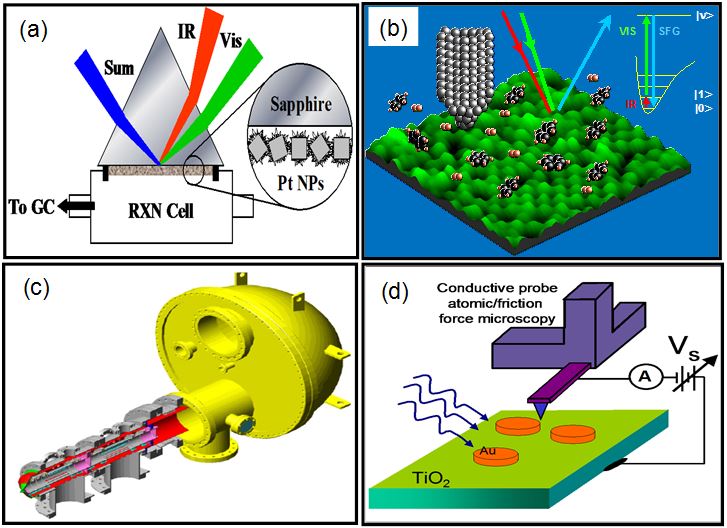
Figure 3 Schemes of high-pressure surface apparatus (a) high‐pressure SFG (sum frequency generation) vibration spectroscopy, (b) ambient -pressure STM, (c) ambient pressure XPS, and (d) in situ AFM.
Energy Dissipation and Conversion at Surfaces
The fundamental aspects of energy dissipation and conversion at surfaces are long-standing questions in surface science, and they have huge implications in energy-related applications. Energy dissipation at surfaces and interfaces is mediated by excitation of elementary processes, including phonons and electronic excitation, once the external energy is delivered to the surface during exothermic chemical processes (Figure 4). The source of external energy can be photons, an electron beam, chemical processes, or mechanical processes. For example, electronic excitation in exothermic catalytic reactions involves the flow of hot electrons with 1–3 eV of energy (assuming that most of the chemical energy is converted to electron flow). Through non-adiabatic electronic excitation, these hot electrons move into the bulk of the metal catalyst, and eventually dissipate energy and turn into low-energy electrons through lattice atom relaxation within the length scale of the electron mean free path (3–10 nm range) and a lifetime of 10 femtoseconds. Understanding the basic mechanism that mediates energy dissipation on surfaces is a key focus of this institute.
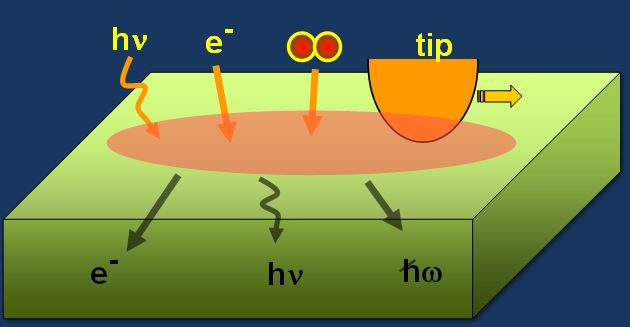
Figure 4 Scheme depicting energy dissipation at surfaces.